Gout is the most common cause of inflammatory arthritis, and its epidemiology worldwide points to an increase in incidence and prevalence in both developed and developing countries6.
Gout is regarded as a prototypical inflammatory disease driven by activation of the innate immune system. Gout has also been termed an autoinflammatory disease, however, this classification is misleading as, unlike hereditary autoinflammatory diseases, the acute trigger of gout is MSU crystals. Uric acid itself is an endogenous and ubiquitous metabolite that is not considered to be pro-inflammatory, and MSU crystal formation is required to provoke clinically observed inflammation.
The study of the underlying mechanisms of gouty inflammation has led to remarkable insights into the control of the inflammasome and pro-inflammatory cytokine release6.
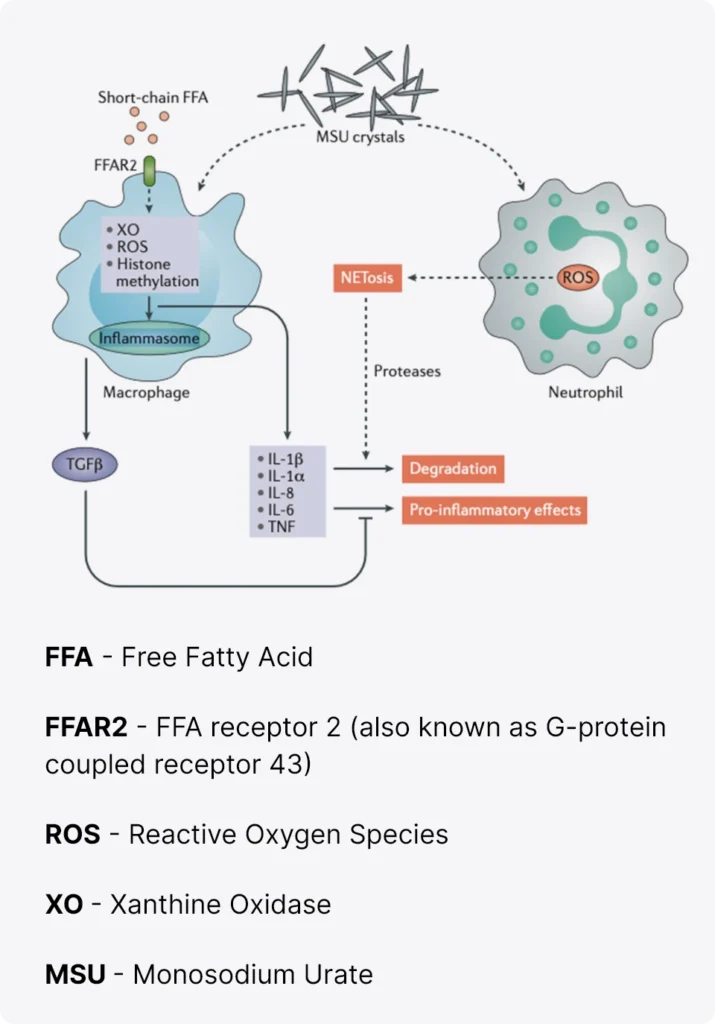
When there’s an excess of uric acid in the blood, it can cause urate crystals to form. These microscopic crystals can sMSU crystals are detected by innate immune cells such as macrophages, monocytes or neutrophils that respond and produce active IL‑1β. IL‑1β signals through the IL‑1 receptor complex, composed of IL‑1 receptor type 1 (IL‑1R1) and its cofactor IL‑1 receptor accessory protein (IL‑1RAcP), leading to recruitment of the adaptor protein MyD88. The expression of IL‑1R1 is widespread, on leukocytes as well as on endothelial and synovial cells. IL‑1R1 expression results in the recruitment of effector proteins such as the IL‑1 receptor-associated kinases (IRAKs) (mostly IRAK4) and TNF receptor-associated factor 6 (TRAF6). This protein recruitment triggers the IκB kinase (IKK) complex and leads to the phosphorylation and degradation of the NF‑κB inhibitor IκB. Activation of NF‑κB turns on the transcription of cytokines and neutrophil-recruiting chemokines, which will amplify the response and initiate a complex inflammatory cascade6.
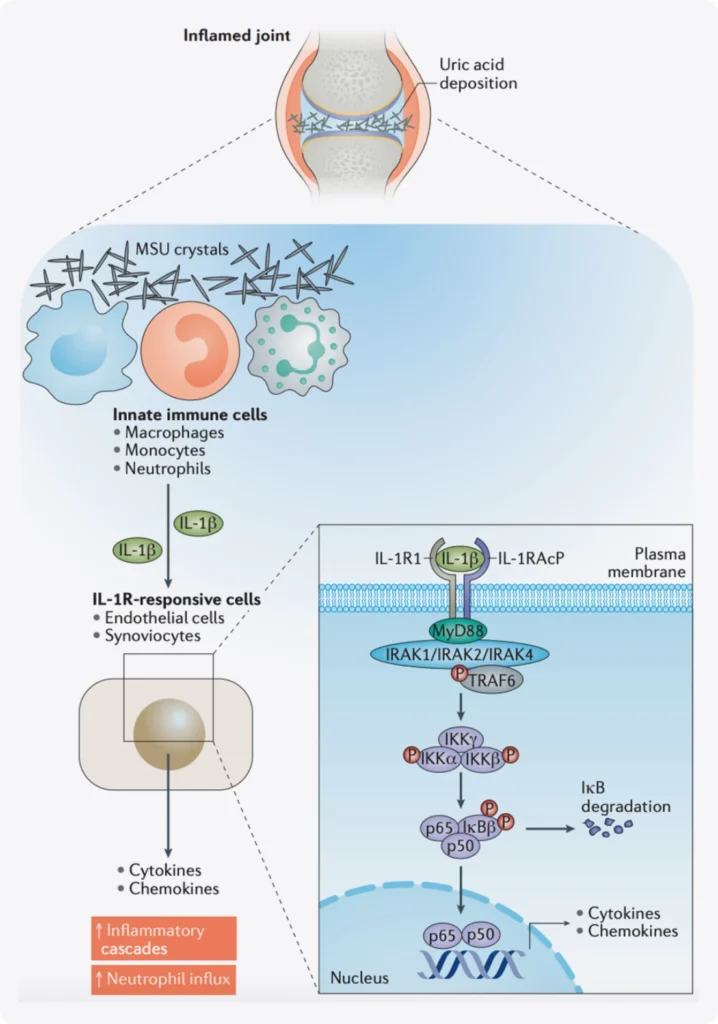
Multiple regulatory pathways influence the acute inflammatory response to monosodium urate (MSU) crystals.
The interaction between macrophages and neutrophils is important in the regulation of the acute inflammatory response.
Modulators of NLRP3 inflammasome activation,including acetate, omega-3 fatty acids and antioxidants, can dampen IL-1β-release.
High concentrations of neutrophils will also favour the formation of neutrophil extracellular trap (NET) aggregates and NETosis, which contain proteases capable of degrading inflammatory cytokines.
Finally, the release of transforming growth factor β (TGFβ) by macrophages also acts as a brake on the inflammatory response.

How to manage
inflammation?